Message from ASNC’s President

Lawrence Phillips, MD, MASNC
Dear Colleagues,
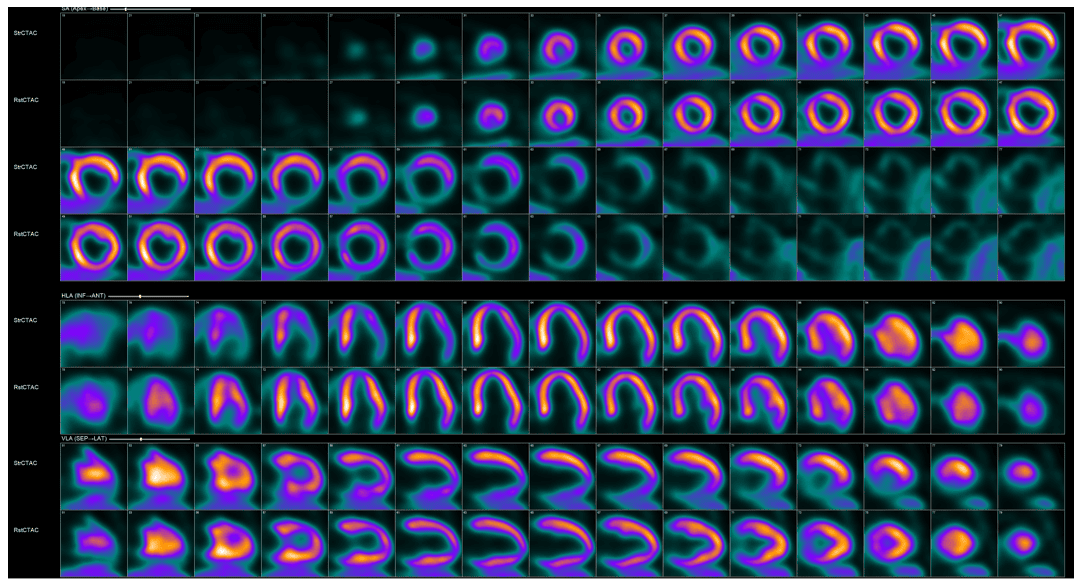
On Friday, Sept. 27, the U.S. Food and Drug Administration (FDA) announced its approval of F-18 flurpiridaz (Flyrcado) for use in adult patients with known or suspected coronary artery disease to evaluate for myocardial ischemia and infarction. This is news the field has been eagerly awaiting since the results of the AURORA trial (International Study to Evaluate Diagnostic Efficacy of Flurpiridaz [18F] Injection PET MPI in the Detection of Coronary Artery Disease) were published in October 2023.
Flurpiridaz is the first new perfusion radiopharmaceutical approved by the FDA in nearly 30 years. Its benefits include higher myocardial extraction and a longer half-life than all currently available PET perfusion radiopharmaceuticals, an advantage that will allow unit dosing, which we predict will expand patients’ access to cardiac PET imaging.
The availability of F18-flurpiridaz adds an important new tool to nuclear cardiology’s already robust armamentarium while helping to usher in an exciting new era for our field. There are many more innovations and advancements on the horizon. ASNC is committed to keeping you current and providing context about what new developments mean for our labs, our field, and our patients. I encourage you to view ASNC’s upcoming programs and make plans to participate. Stay tuned for updates.
Best regards,
Lawrence Phillips, MD, MASNC
ASNC President
ASNC Leaders Featured in F-18 Flurpiridaz FDA Approval Press Coverage
ASNC leaders and educational program faculty were among those interviewed by journalists from AuntMinnie.com, Cardiology Today/Healio, Cardiovascular Business, DotMed, and HealthCareBusiness.
Read Dr. Phillips interview with TCTMD here.
Article Type
News & Announcements
Category
Advocacy, Education, Publications, Research
Related Posts
ASNC2025 News! Winners of New Fellows-in-Training Poster and Case Awards
In celebration of nuclear cardiology’s vibrant community of early-career investigators, the ASNC2025…
ASNC Announces Competitors for 2025 Barry L. Zaret Young Investigator Awards
At ASNC2025, the following early-career investigators will compete for cash prizes in…
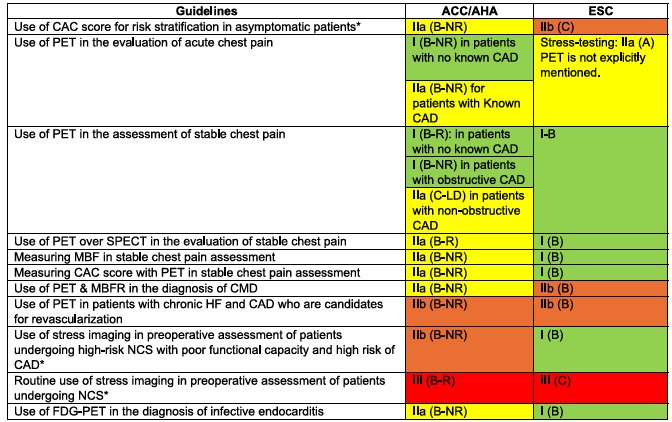
Cardiac PET in the Guidelines: The Update Your Practice Needs Now
Cardiac PET and PET/CT have earned new indications in recently published guidelines…