ASNC is your source for the latest information emerging from the field of nuclear cardiology and cardiac imaging.
From our flagship scientific journal to our weekly newsletter, ASNC is dedicated to delivering high-quality research, news, and updates from the field.

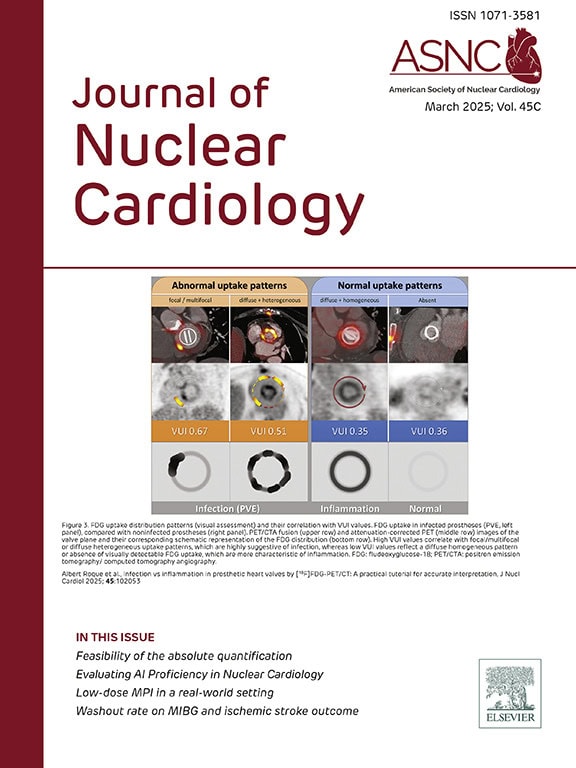
Journal of Nuclear Cardiology
The Journal of Nuclear Cardiology (JNC) is the only journal in the world devoted to this dynamic and growing subspecialty. The JNC is the official peer-reviewed publication of ASNC featuring the latest research findings and clinical developments in nuclear cardiology and related fields.
Latest Journal Issue
Read the latest issue of the Journal of Nuclear Cardiology (JNC). Full access to the JNC is available with an ASNC Membership. Join or renew today!
Nov 2025,
Vol. 53
The November issue explores the debate over functional versus structural imaging in patients with suspected coronary artery disease. In his Editor’s Message, Marcelo F. Di Carli argues that a third approach—one that combines both modalities and incorporates artificial intelligence—may ultimately prevail as the key to understanding ischemia in all its forms. New science in this issue offers proof points for that perspective, illustrating how SPECT or PET, CT, and AI can be synergized into a data-rich framework for cardiovascular care.
Oct 2025,
Vol. 52
The October issue examines the possibility that SPECT could help lead the next generation of quantitative nuclear cardiology. In his Editor’s Page, Marcelo F. Di Carli outlines the technical limitations that must be overcome, particularly with current 99mTc-labeled tracers, to enable myocardial blood flow quantification and improve detection of moderate ischemia in an era of nonobstructive coronary artery disease. Two original studies underscore what he describes as a “continuum of progress,” including preclinical and first-in-human evaluation of a novel SPECT tracer and an analysis of CZT-SPECT performance for myocardial blood flow and flow reserve quantification.
Sept 2025,
Vol. 51
The September issue highlights the impact of best-practice SPECT myocardial perfusion imaging on laboratory efficiency, patient safety, and long-term prognosis. One large observational study demonstrates how combining attenuation correction with prone positioning can substantially increase stress-only imaging, reducing the need for rest studies and significantly lowering radiotracer dose. A second investigation shows that accounting for post-test revascularization uncovers the true prognostic value of stress MPI, with important implications for both clinical decision-making and outcomes research.
New JNC Podcast
The JNC CardioConnect podcast links experts, insights, and innovations in nuclear cardiology. Join Editor-in-Chief Marcelo F. Di Carli, MD, MASNC, and authors as they discuss important articles from the Journal of Nuclear Cardiology (JNC).

ASNC Updates
Access the latest news and updates from ASNC. Search by category of interest, date, and more.
Manufacturer Reports PYP, HMDP Supplies Expected to Rebound in First Quarter 2026
On Jan. 12, ASNC reported that there have been shortages of both…
Medicare Policy Changes Are Impacting Nuclear Cardiology Now
On Jan. 1, physician practices across the U.S. began feeling the effects…
PYP and HMDP Supply Issues Expected in Early 2026
ASNC has confirmed reports that supply disruptions with both Tc-99m pyrophosphate (PYP)…
Receive ASNC SmartBrief
Sign up to receive ASNC's bi-weekly round-up of news and information related to nuclear cardiology and cardiovascular imaging.

ASNC Interview Series
Explore the latest topics in nuclear cardiology through video conversations with luminaries and key leaders in the field.
Advertising
ASNC is where your marketing investment reaches the right audience and achieves optimal return.