ASNC is your source for the latest information emerging from the field of nuclear cardiology and cardiac imaging.
From our flagship scientific journal to our weekly newsletter, ASNC is dedicated to delivering high-quality research, news, and updates from the field.

Journal of Nuclear Cardiology
The Journal of Nuclear Cardiology (JNC) is the only journal in the world devoted to this dynamic and growing subspecialty. The JNC is the official peer-reviewed publication of ASNC featuring the latest research findings and clinical developments in nuclear cardiology and related fields.
Read the latest issue of the Journal of Nuclear Cardiology (JNC). Full access to the JNC is available with an ASNC Membership. Join or renew today!
The May issue showcases the field’s evolution with studies on vascular aging, hybrid imaging, and AI in patient education. The Editor’s Choice highlights a 99mTc-HMDP SPECT/CT myocardium-to-blood ratio for the detection of ATTR cardiomyopathy , with an editorial pointing to a future of more precise, quantitative imaging. Additional research examines myocardial flow reserve by diabetes status, PET/MRI for scar detection, and ChatGPT’s accuracy in answering patient questions. A review on fibroblast activation imaging and a debate on amyloidosis screening complete the issue.
The April issue highlights the potential of nuclear cardiology to improve assessment and treatment of peripheral artery disease (PAD). The Editor’s Choice study, supported by an ASNC/IANC Research Fellowship grant, explores post-exercise PET imaging of skeletal muscle perfusion to evaluate PAD severity, with an editorial noting its contribution to the growing body of PAD imaging research. A state-of-the-art review examines how lessons from coronary artery disease could guide PAD care. Additional content includes a phase I study of a fatty acid PET tracer, FDG PET imaging for cardiac sarcoidosis, and reflections on innovation in the field.
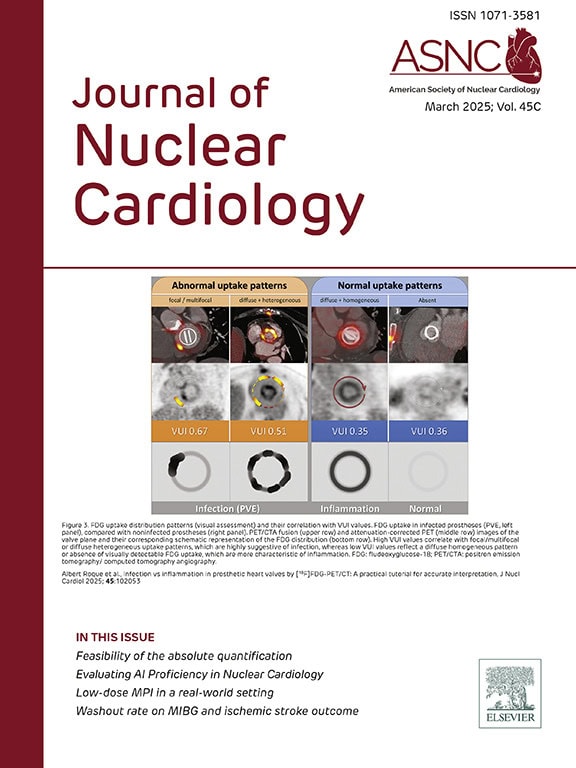
The March issue features a President’s Message from Dr. Panithaya Chareonthaitawee on raising awareness of amyloidosis. A featured study challenges four large language models (LLMs) with ASNC’s Board Exam practice questions, exploring AI’s potential in image interpretation and clinical reasoning. Other highlights include the I-NERVE study on [123I]-MIBG SPECT/CT in wild-type transthyretin amyloidosis, the accuracy of low-dose myocardial perfusion imaging, a tutorial on diagnosing infection vs inflammation with [18F]FDG-PET/CT, and more.
The JNC CardioConnect podcast links experts, insights, and innovations in nuclear cardiology. Join Editor-in-Chief Marcelo F. Di Carli, MD, MASNC, and authors as they discuss important articles from the Journal of Nuclear Cardiology (JNC).

Access the latest news and updates from ASNC. Search by category of interest, date, and more.
‘One Big Beautiful Bill’ Signed into Law, Includes Pay Bump for Physicians
President Trump signed H.R. 1, the One Big Beautiful Bill Act, into…
ASNC and EANM Hosting ICNC’26 in Berlin, Germany
ICNC’26 – the International Conference on Nuclear Cardiology – will be held…
ASNC2025 News! Winners of New Fellows-in-Training Poster and Case Awards
In celebration of nuclear cardiology’s vibrant community of early-career investigators, the ASNC2025…
Sign up to receive ASNC's bi-weekly round-up of news and information related to nuclear cardiology and cardiovascular imaging.

ASNC Interview Series
Explore the latest topics in nuclear cardiology through video conversations with luminaries and key leaders in the field.
Advertising
ASNC is where your marketing investment reaches the right audience and achieves optimal return.