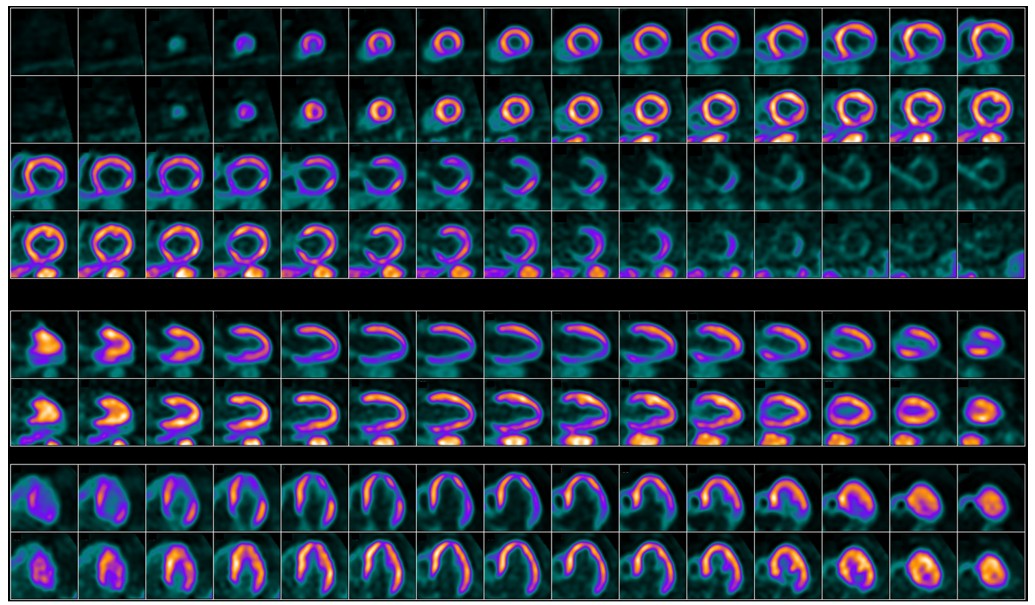
Fairfax, VA (September 18, 2025)—The American Society of Nuclear Cardiology (ASNC), Society of Nuclear Medicine and Molecular Imaging (SNMMI), European Association of Nuclear Medicine (EANM), and American College of Nuclear Medicine (ACNM) have issued a new clinical guideline for 18F-flurpiridaz PET myocardial perfusion imaging (MPI) and blood flow quantitation. The guideline, co-published in the Journal of Nuclear Cardiology and The Journal of Nuclear Medicine, is intended to assist nuclear cardiology practitioners in the optimal application of 18F-flurpiridaz PET for MPI, including recommendations for patient selection, imaging protocols, and interpretation of results.
Coronary artery disease (CAD) is the leading cause of death worldwide and is responsible for the hospitalization, functional decline, and reduced quality of life for millions of patients each year. Early and accurate diagnosis and risk stratification of CAD are essential for guiding treatment and improving patient outcomes. MPI is a cornerstone of non-invasive assessment of myocardial ischemia.
MPI can be performed with SPECT or PET. 18F-flurpiridaz is a novel PET radiotracer that has demonstrated favorable imaging and physiological properties compared to conventional SPECT agents. Clinical studies have shown that it provides superior image quality, improved diagnostic accuracy, and greater sensitivity in detecting clinically significant coronary artery stenosis compared with conventional SPECT agents. It is particularly valuable in patients for whom SPECT may be less effective, such as those with obesity or small left ventricles.
“Multiple phase II and two phase III clinical trials have further established the utility of 18F-flurpiridaz as a valuable PET radiotracer for the non-invasive evaluation of CAD,” noted René R. Sevag Packard, MD, PhD. “Owing to its favorable molecular imaging characteristics and extended half-life, 18F-flurpiridaz is poised to become a valuable agent for cardiac stress PET imaging, offering clinicians a robust tool for the evaluation of CAD.”
The guideline includes information on clinical indications, contraindications, and considerations for 18F-flurpiridaz PET. It addresses qualifications and responsibilities of personnel performing cardiac stress testing using 18F-flurpiridaz, protocol (and potential protocol modifications), biodistribution and dosimetry, and the 18F-flurpiridaz PET workflow. Technologist considerations are also included.
“This document is designed to promote high-quality, safe, and effective nuclear medicine practice in the evaluation of CAD,” emphasized the authors. “Our hope is that the guideline enhances clinical decision-making, standardizes clinical practice, improves diagnostic accuracy, and optimizes resource utilization to ensure safety and improve patient outcomes.”
ASNC, SNMMI, EANM, and ACNM periodically define new procedure standards/practice guidelines for nuclear medicine practice to help advance the science of nuclear medicine and to deliver effective and safe medical care to patients. Each standard/guideline, representing a policy statement by these groups, undergoes a thorough consensus process in which it is subjected to extensive review.
Article Type
Press Release
Category
Guidelines & Quality
Related Posts
Immediate Impact! ASNC Statement Driving Cardiac PET Adoption Across the United States
“ASNC’s Clinical Indications for PET statement got the attention of our hospital…
2026 Heart Month Offers
Celebrate Heart Month with ASNC!💗 In recognition of Heart Month, you are…
Cardiac PET, If Available, Is Now the Preferred Test for Evaluating CAD in All Patients
“Based on the clinical and scientific evidence now available, there are no…