Sponsored by Alnylam Pharmaceuticals

Early Diagnosis and Treatment Put Age-Expected Longevity within Reach for Patients with ATTR-CM
If you have attended a CME program addressing cardiac amyloidosis in the past year, there’s a good chance you’ve seen experts discussing this figure:
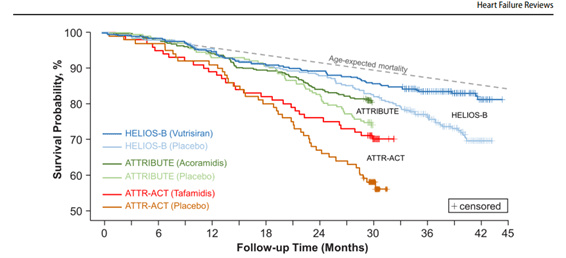
Time to All-Cause Mortality Compared Among Trials in ATTR-CM. From Girard AA, Sperry BW. Contextualizing the results of HELIOS-B in the broader landscape of clinical trials for the treatment of transthyretin cardiac amyloidosis. Heart Fail Rev. 2025;30,69-73. Figure reprinted with permission of the author.
“This beautiful figure shows that we are entering an era where patients should expect not to die from cardiac amyloidosis but rather with cardiac amyloidosis,” says Michelle M. Kittleson, MD, PhD, heart failure/transplant cardiologist at Cedars-Sinai Medical Center in Los Angeles, California. “It’s incredibly exciting.”
“…We are entering an era where patients should expect not to die from cardiac amyloidosis but rather with cardiac amyloidosis.”
- Michelle M. Kittleson, MD, PhD
Created by Brett W. Sperry, MD, FASNC, and published in Heart Failure Reviews (1), the graph overlays the survival curves for the treatment and placebo arms of the landmark trials for the 3 currently available amyloid medications with the age-expected mortality of individuals their age in the U.S. general population.
Among the trials was HELIOS-B, the randomized, placebo-controlled ATTR-CM trial that found vutrisiran significantly lowered the risk of all-cause death and recurrent cardiovascular events, reduced hospitalizations, and preserved functional capacity and quality of life as measured on the 6-minute walk test and the Kansas City Cardiomyopathy Questionnaire-Overall Summary.(2,3)
“HELIOS-B is the most contemporary trial we have in terms of the ATTR-CM patient population. I wanted to see the age-expected life span for HELIOS-B patients alongside actuarial data for other 77-year-olds,” explains Dr. Sperry, an advanced heart failure and imaging cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Missouri. “The chart shows that in the HELIOS-B treatment arm, patients with transthyretin amyloidosis who were treated with vutrisiran, or in some cases vutrisiran and tafamidis, had mortality rates approaching what was expected for their age.”
New Analysis Hints at Why
The field of cardiac amyloidosis has progressed at remarkable speed, but there are still many unanswered questions, among them how clinicians could evaluate a patient’s response to therapy and then, based on the evaluation, adjust treatment. Now, the results of a HELIOS-B substudy(4) offer what Dr. Kittleson calls “a tantalizing glimpse into a future in which therapeutic decision-making may be guided by an accurate assessment of prognosis and response to therapy.”(5)
Karola S. Jering, MD, and colleagues analyzed echocardiographic data from HELIOS-B starting at baseline and continuing through month 30. They found that worse left and right ventricular systolic and diastolic function at baseline were independently associated with higher risk of the trial’s composite endpoint of all-cause death and recurrent cardiovascular events as well as with all-cause death alone. By 12 months, vutrisiran lowered E/e’, thus improving diastolic function, and, by 18 months, had attenuated declines in left ventricular and right ventricular systolic function. Worsening of left ventricular and right ventricular systolic function at 18 months was associated with higher risk of all-cause death and recurrent cardiovascular events.
“This substudy hints at some of the mechanisms for how vutrisiran may work to facilitate clinical benefits,” says Dr. Jering, a cardiovascular disease specialist at Mass General Brigham in Boston, Massachusetts. “As more amyloid fibrils accumulate in the heart, we generally see thickening of the myocardium, leading to worsening diastolic function over time. We also look specifically at global longitudinal strain because it can detect even subclinical changes in left ventricular function more so than left ventricular ejection fraction. In HELIOS-B, vutrisiran attenuated increases in left ventricular wall thickness as well as declines in left ventricular and right ventricular systolic function, and it even improved diastolic function, which we haven’t seen before with the other agents.”
“In HELIOS-B, vutrisiran attenuated increases in left ventricular wall thickness as well as declines in left ventricular and right ventricular systolic function, and it even improved diastolic function, which we haven’t seen before with the other agents.”
- Karola S. Jering, MD

Dr. Jering wasn’t surprised by the results of the echocardiography analysis. “Across HELIOS-B, you see favorable changes in biomarkers, including NT-proBNP and troponin, and you see clinical benefits, including improved quality of life, reduced cardiovascular events, and mortality,” she says. “The echo findings show favorable changes in cardiac structure and function with vutrisiran, and they are complementary to its effects on hard clinical outcomes. It all nicely travels in the same direction and is supportive of vutrisiran’s favorable effects.”
Now What? Optimal Outcomes Still Start with Early, Accurate Diagnosis
Dr. Sperry says he welcomes “rigorous data showing less worsening of echo parameters … it’s what we are hoping for – stability of the disease symptoms and of the echo findings as far as structure and function of the heart.”
He and co-author Andrew A. Girard, MD, liken amyloidosis to a flooded basement, where the imperative is turning off the running water and then cleaning up the mess. Vutrisiran, the only FDA-approved TTR silencer for ATTR-CM, leverages RNA interference to turn off the water – in other words to slow or stop amyloid deposition.(1)
An important key to success with vutrisiran as well as the other ATTR-CM medications is starting therapy early. Each of the physicians we talked to for this article – Dr. Kittleson, Dr. Sperry, and Dr. Jering – pointed out that clinical outcomes are improving over time due to greater awareness of the condition, better screening, earlier diagnosis, and earlier treatment.
"It comes down to all of us – cardiologists and non-cardiologists. We have to be on the lookout for signs and get patients evaluated early and appropriately using nuclear scintigraphy as a first-line diagnostic strategy. And then we need to get them on good, disease-modifying treatment as soon as possible.”
- Brett W. Sperry, MD, FASNC

“With early treatment, we may be able to turn ATTR-CM into a chronic disease that patients have to manage, as opposed to something that will progress and worsen both their quality of life and their longevity,” Dr. Sperry says. “It comes down to all of us – cardiologists and non-cardiologists. We have to be on the lookout for signs and get patients evaluated early and appropriately using nuclear scintigraphy as a first-line diagnostic strategy. And then we need to get them on good, disease-modifying treatment as soon as possible.”
References
- Girard AA, Sperry BW. Contextualizing the results of HELIOS-B in the broader landscape of clinical trials for the treatment of transthyretin cardiac amyloidosis. Heart Fail Rev. 2025;30:69-73.
- Fontana M, Berk JL, Gillmore JD, et al. Vutrisiran in patients with transthyretin amyloidosis with cardiomyopathy. N Engl J Med. 2024;392:33-44.
- Witteles RM, Garcia-Pavla P, Damy T, et al. Vutrisiran improves survival and reduces cardiovascular events in ATTR amyloid cardiomyopathy: HELIOS-B. J Am Coll Cardiol. 2025;85(20)1959-70.
- Jering KS, Fontana M, Skali H, et al. Effects of vutrisiran on cardiac function and outcomes in patients with transthyretin amyloidosis with cardiomyopathy. J Am Coll Cardiol. 2025;86(6):444-55.
- Kittleson MM. Structure, function, and outcomes in transthyretin amyloid cardiomyopathy. J Am Coll Cardiol. 2025;86(6):456-8.
This article is sponsored by Alnylam Pharmaceuticals. The writing was undertaken by ASNC, independent from the sponsor.