Precision meets flexibility
Now, you can have both. The precision of cardiac PET over SPECT joins the flexibility enabled by Flyrcado to increase access, diagnostic accuracy, and options for your cardiac imaging team.1-6
Precision meets possibility with Flyrcado
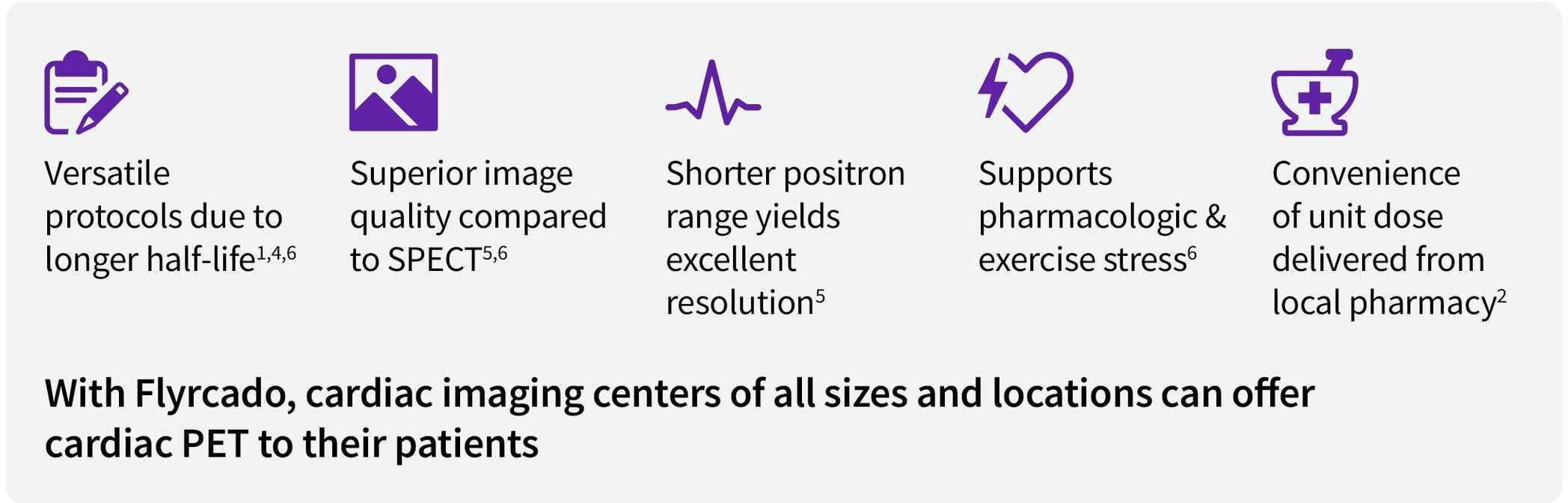
Enhance operational flexibility in cardiac PET
With delivery of on-demand unit doses up to five days a week, Flyrcado opens the door to cardiac PET for more imaging centers. 1,4-6
Comprehensive on-board support
As the industry’s singular provider of both advanced imaging technologies and proprietary radiotracers, our team of multidisciplinary GE HealthCare experts is here to support all your needs. To learn more about our offerings or to schedule training, please visit our website or Contact Us
Be Flyrcado Forward
We’re here to support you every step of the way as you prepare to open the door to cardiac PET. Flyrcado Forward is a comprehensive program of continued guidance, tailored resources, and expert-backed support to help your site of care expand access to cardiac PET to more patients in more places.
Sign up today to unlock access to exclusive content and learning opportunities.

For more information about Flyrcado, and to view full Prescribing Information, please visit our website.
References
- Maddahi J, Packard RRS. Cardiac PET perfusion tracers: current status and future directions. Semin Nucl Med. 2014;44(5):333-343.
- Flyrcado (flurpiridaz F 18 injection) . Full Prescribing Information. Marlborough, MA: GE HealthCare.
- Maddahi J, Agostini D, Bateman TM, et al. Flurpiridaz F-18 PET Myocardial perfusion imaging in patients with suspected coronary artery disease. J Am Coll Cardiol. 2023;82(16):1598-1610.
- Patel KK, Singh A, Bateman TM. The potential of F-18 flurpiridaz PET/CT myocardial perfusion imaging for precision imaging. Curr Cardiol Rep. 2022;24(8):987-994.
- Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging. 2017;33(7):1021-1031.
- Iskandrian AE, Dilsizian V, Garcia EV, et al. Myocardial perfusion imaging: lessons learned and work to be done update. J Nucl Cardiol. 2018;25(1):39-52.
- Bourque JM. Cardiac PET perfusion imaging: AURORA lights the way to a new era. J Am Coll Cardiol. 2023;82(16):1611-1613.
Important Safety Information
Indications and Usage
FLYRCADO is a radioactive diagnostic drug indicated for positron emission tomography (PET) myocardial perfusion imaging (MPI) under rest or stress (pharmacologic or exercise) in adult patients with known or suspected coronary artery disease (CAD) to evaluate for myocardial ischemia and infarction.
Contraindications
None
Warnings and Precautions
- Risk associated with exercise or pharmacologic stress: Patients evaluated with exercise or pharmacologic stress may experience serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, stroke, and seizure. Perform stress testing in the setting where cardiac resuscitation equipment and trained staff are readily available. When pharmacologic stress is selected as an alternative to exercise, perform the procedure in accordance with the pharmacologic stress agent’s prescribing information.
- Radiation risks: FLYRCADO contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling to minimize radiation exposure to patients and health care providers. Advise patients to hydrate before and after administration and to void.
Adverse Reactions
- Most common adverse reactions occurring during FLYRCADO PET MPI under rest and stress (pharmacologic or exercise) (incidence ≥2%) are dyspnea, headache, angina pectoris, chest pain, fatigue, ST segment changes, flushing, nausea, abdominal pain, dizziness, and arrhythmia.
To report SUSPECTED ADVERSE REACTIONS, contact GE HealthCare at 800-654-0118 (option 2 then option 1) or by email at GPV.drugsafety@gehealthcare.com or FDA at 800-FDA-1088 or www.fda.gov/medwatch
Please see the full Prescribing Information for additional important safety information.
© 2025 GE HealthCare
Flyrcado is a trademark of GE HealthCare. GE is a trademark of General Electric Company used under trademark license.
September 2025 | JB12399US